Researchers from the University of Birmingham, U.K., and Duke University, U.S., have created a new family of polymers from sustainable sources that retain all of the qualities of common plastics, but are also degradable and mechanically recyclable.
The scientists used sugar-based starting materials rather than petrochemical derivatives to make two new polymers, one that is stretchable like rubber and another which is tough but ductile, like most commercial plastics.
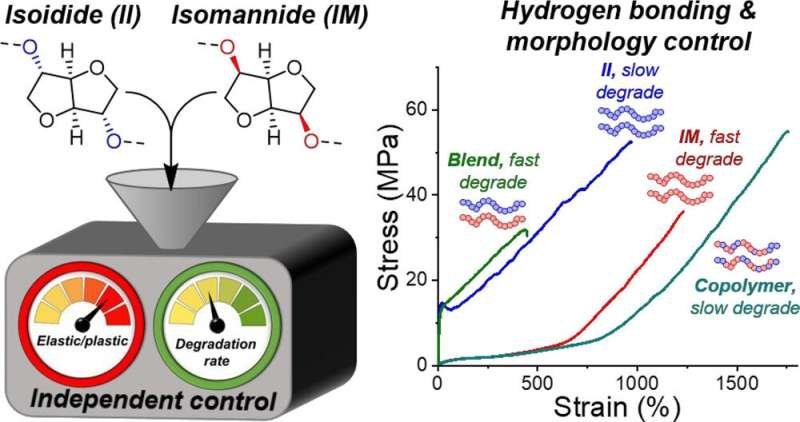
The researchers made the new polymers using isoidide and isomannide as building blocks. Both these compounds are made from sugar alcohols and feature a rigid ring of atoms (picture). The researchers found that the isoidide-based polymer, showed a stiffness and malleability similar to common plastics, and a strength that is similar to high grade engineering plastics such as Nylon-6. Despite isoidide and isomannide only differing by the 3D spatial orientation of two bonds, known as stereochemistry, the isomannide-based material had similar strength and toughness but also showed high elasticity, recovering its shape after deformation. Notably, the materials retained their excellent mechanical properties following pulverization and thermal processing, which is the usual method for mechanically recycling plastics.
Cutting-edge computational modeling simulated how the polymer chains pack and interact to produce such different =”https://phys.org/tags/polymer/” rel=”tag”>polymer properties. The unique 3D shapes of the sugar derivatives facilitate different movements and interaction of the long chains causing the huge difference in physical properties that was observed.
By creating copolymers that contain both isoidide and isomannide units, the researchers found that they could control the mechanical properties and degradation rates independently of one another. Hence, this system opens the door to using the unique shapes of sugars to independently tune the degradability for a specific use without significantly altering the properties of the material.
The chemical similarity of the polymers means that, unlike a lot of current commodity plastics, they can be blended together to yield materials with comparable or improved properties.
Dr. Josh Worch, from Birmingham's School of Chemistry, and a co-author in the research, said: "The ability to blend these polymers together to create useful materials, offers a distinct advantage in recycling, which often has to deal with mixed feeds."
Dr. Connor Stubbs, also from Birmingham's School of Chemistry, added: "Petrol based plastics have had decades of research, so catching up with them is a huge challenge. We can look to the unique structures and shapes that biology have to offer to create far better plastics with the same expanse of properties that current commercial plastics can offer."
Duke University professor Dr. Matthew Becker said: "Our findings really demonstrate how stereochemistry can used as a central theme to design sustainable materials with what truly are unprecedented mechanical properties."
Professor Andrew Dove, who led the research team from Birmingham, noted: "This study really shows what is possible with sustainable plastics. While we need to do more work to reduce costs and study the potential environmental impact of these materials, in the long term it is possible that these sorts of materials could replace petrochemically-sourced plastics that don't readily degrade in the environment."
A joint patent application has been filed by University of Birmingham Enterprise and Duke University. The researchers are now looking for industrial partners who are interested in licensing the technology.
Source: =”https://phys.org/news/2022-01-sweet-breakthrough-scientists-recyclable-plastics.html” target=”_blank” rel=”noopener”>www.phys.org







Leave a reply